Transcatheter Mitral Valve Repair and Replacement Market Opportunity Analysis 2018 – 2026
Transcatheter mitral valve repair and replacement methods are gaining significant traction due to advances in valve therapy. Transcatheter Mitral Valve Replacement (TMVR) can be used as an alternative to open-heart surgery and is used in treating mitral valve stenosis, mitral valve prolapse, and mitral valve regurgitation.
Transcatheter Mitral Valve Repair and Replacement Market Drivers
As per the American Heart Association estimates in 2017, around 19.1 million death occur annually worldwide due to various cardiovascular diseases. Furthermore, as per the United Nations (UN) estimates 2017, the geriatric population in the U.S. is expected to reach over 72 million by 2022. The growth of this target population coupled with increasing risk of cardiovascular diseases is expected to drive the growth of the market.
Click To Continue Reading on Transcatheter Mitral Valve Repair and Replacement Market
The global transcatheter mitral valve repair and replacement devices market is expected to witness growth, owing to continuous technological advancements such as developing multiple use valves. For instance, in June 2017, Edwards Lifesciences received FDA approval for its SAPIEN 3 transcatheter prosthetic heart valve, indicated for placement within both mitral valves and aortic valves.
Increase in number of TMVR pipeline devices for treatment of mitral valve abnormalities are also expected to propel growth of TMVR market. For instance, a randomized clinical trial of Medtronic Intrepid Transcatheter Mitral Valve Replacement System, sponsored by Medtronic Cardiovascular is ongoing for treating mitral valve regurgitation and is estimated to complete in October 2025.
Transcatheter Mitral Valve Repair and Replacement Market Restraints
Stringent government regulations as well as high cost of mitral valve surgeries are major factors restraining growth of the market. For instance, according to an article published in National Chemical Biology Institute (NCBI), in September 2015, mean cost of TMVR surgery was US$ 42,806. According to Times of India March 2012, the cost of TMVR procedure in India was US$ 29,350 which include the estimated cost of valve US$ 21,500.
Transcatheter Mitral Valve Repair and Replacement Market – Regional Analysis
On the basis of region, the transcatheter mitral valve repair and replacement market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa.
North America is projected to dominate transcatheter mitral valve repair and replacement market, owing to high prevalence of cardiac disease. For instance, in the U.S., heart disease is a leading cause of death, accounting for around 375,000 deaths annually. According to ClinicoEconomics and Outcomes Research 2016, Mitral regurgitation is the most frequent valve disease in the U.S. affecting nearly 1 in 10 people of 75 years or older.
Asia Pacific region is expected to witness significant growth, owing expansions of manufacturing facilities by market players. For instance, Medtronic, Inc. in March 2011, opened new manufacturing facility in Singapore to meet increasing demand for cardiac devices in region.
Request a sample copy of this report: https://www.coherentmarketinsights.com/insight/request-sample/2189
Key Vendors:
Abbott Laboratories, Biotronik Private Limited, Colibri Heart Valve, Edward Lifesciences Corporation, HLT Medical, LivaNova Plc, Medtronic Plc, Neovasc Inc., and NeoChord.

Inoculating Turntables Market Outlook and Opportunity Analysis 2018–2026
Inoculating turntables are small devices used for easy and even spreading of inoculum on the substrate, during culture media preparation and straining. Inoculating turntables turn around petri dish on a concentric point using ball bearings, which ensures smooth and effortless spinning for faster and more accurate inoculation. Inoculating turntables are of two types based on the mode of force used to turn the platform — hand-operated and electronic. Electronic turntables offers different rpm settings and a hand or leg operated pedal to on and off the rotations. Increasing number of end users of this product, is expected to be major factor driving the inoculation turntable market growth during the forecast period.
Click To Continue Reading on Inoculating Turntables Market
Inoculating Turntables Market Dynamics
For instance, to assess drug efficacy on any bacteria or microbes, the culture media plays a vital role in bacteria production. The involvement or usage of inoculating turntable helps in dense growth of bacterial/microbial colony. Inoculating turntables are commonly used in culture in research laboratories, majorly for antiseptic and antibiotics drugs. For instance, according to Lancet Infectious Diseases Commission, 2014, the consumption of antibiotic drugs raised gradually to 36% from 2000 to 2010. Additionally, BRICS countries contributed a share of around 75.4% of the growth. Dense antibiotic drug manufacturing by culture media on inoculating turntable, is a major factor driving growth of Inoculating turntable market. Moreover, all such factors favor an opportunity-rich growth for the inoculating turntable market over the next couple of decades.
Inoculating Turntables Market Taxonomy
Based on product type, the inoculating turntables market is segmented into:
- Manual/ Hand-operated
- Sensor based
Based on platform size, the inoculating turntables market is segmented into:
- 100 mm
- 150 mm
- Others
Based on end users, the inoculating turntables market is segmented into:
- Culture laboratories
- Research and Development Institutes
- Clinical Research Organizations
- Others
Inoculating Turntables Market — Regional Analysis
Regional segmentation of inoculating turntables market by Coherent Market Insights includes North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. North America inoculating turntable market held the dominant position in the year 2017 and the region is expected to retain its position over the forecast period. This is owing to rising inoculation turntable application and presence of major pharmaceutical players in the region. However, Asia Pacific inoculating turntable market is expected to exhibit strong trajectory growth. This is owing to fast developing biotechnology sectors in emerging countries such as India and China. For instance, according to India Brand Equity Foundation, 2017 report, India holds 12th position in biotech destination in the world and third position in the Asia Pacific region.
Request a sample copy of this report:https://www.coherentmarketinsights.com/insight/request-sample/2106
Key Vendors:
Thomas Scientific, Merck & Co., WLD-TEC GmbH, ISOLAB Laborgerate, and Cole-Parmer Instrument Company, LLC.

Disposable Blood Bags Market Trends, and Forecast to 2026
Disposable blood bags are used for collection, storage, transportation, and transfusion of human blood and blood components. These bags are manufactured from bio-compatible PVC, compounded with suitable additives, which is not reactive to blood components. Disposable blood bags replace the use of glass bottles as glass bottles require thorough cleaning, rinsing, and autoclaving procedures as well as there are chances of its breakage at any stage. Furthermore, the use of disposable bags eliminates the possibility of any contamination.
Disposable Blood Bags Market Dynamics
Demand for disposable blood bags are witnessing significant growth owing to rising prevalence of blood related disorders and increasing number of road accidents that require blood transfusion. Blood transfusion is commonly used for supportive care in transplant surgery, cardiovascular surgery, massive trauma, and haematological therapy. According to New York Blood Center (NYBC) 2018 data, car accident and trauma victims may need over 50 red cell transfusions during entire treatment process.
Click To Continue Reading on Disposable Blood Bags Market
Moreover, growing aging population, rising government initiatives, and increasing awareness for blood donation are major factors driving growth of the disposable blood bags market. For instance, according to the WHO report of 2016, approximately 112.5 million units of donated blood are collected annually worldwide. All these factors are expected to give significant thrust to the size of the global disposable blood bags market. However, huge shortfall in blood supplies in low-income countries due to lack of a robust infrastructure for blood collection, distribution, and storage is hampering growth of the disposable blood bags market. In emerging economies, blood is primarily used to treat pregnancy complications and childhood anaemia. According to Safe Blood for Africa, nearly 15% of child mortality in Africa occur due to an inadequate or unsafe supply of blood for transfusion.
Disposable Blood Bags Market Growth Drivers
Rising prevalence of diseases such as cancer and various blood related disorder (anemia, hemophilia, and sickle cell disease) is increasing demand for blood transfusion, which eventually drives growth of the disposable blood bags market. For instance, according to Red Cross Blood Organization facts of 2017 about blood needs, sickle cell disease affects 90,000 to 100,000 people in the U.S. and approximately 1,000 babies are born with the disease each year. Sickle cell patients often require blood transfusions throughout their lives.
Increasing number of cancer incidences worldwide is also a major factor driving the disposable blood bags market’s growth. For instance, according to American Cancer Society, 2018, 1,735,350 new cancer cases and 609,640 cancer deaths are projected to occur in the U.S. by the end of 2018. Majority of these patients will need blood, sometimes daily, during chemotherapy treatment. Furthermore, according to the New York Blood Center, approximately 4.5 million Americans receive blood transfusions each year and 40,000 pints are transfused each day in the country, to save the lives of cancer patients, burn, accident, and trauma victims, mothers delivering babies, newborn babies, transplant patients, surgery patients and others in need.
Disposable Blood Bags Market - Regional Insights
On the basis of region, the global disposable blood bags market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. In 2026, North America is expected to hold the leading position in the global disposable blood bags market, owing to well-established medical and healthcare infrastructure providing timely blood transfusion services. Growing awareness among people for blood donation is also a major factor driving growth of the disposable blood bags market. According to the Red Cross Blood Organization blood supply statistics of 2017, an estimated 6.8 million people in the U.S. donate blood every year.
Asia Pacific disposable blood bags market is expected to grow at the highest rate due to high demand for blood collection bags in the region. Growing healthcare facilities will act as a driver for growth of medical sector in countries such as India. Continuous developments and rising demand for better blood collection technology in the region is expected to boost the disposable blood bags market in the near future.
Request a sample copy of this report: https://www.coherentmarketinsights.com/insight/request-sample/2170
Key Vendors:
AdvacarePharma, Fresenius Kabi, Grifols, HLL Lifecare, Haemonetics Corporation, Macopharma, Neomedic, Poly Medicure, SURU International, Span Healthcare, and TERUMO PENPOL.

Radiopharmaceuticals Market Size, Trend and Outlook 2026
Radiopharmaceuticals are unique medicinal formulations that contain radioisotopes and are used in clinical applications for diagnosis and therapy. The facilities and procedures for production, use, and storage of radiopharmaceuticals are subject to license approval either by national or regional authorities such as Nuclear Regulatory Commission (NRC) of U.S, Atomic Energy Commission (AEC) of U.S., or Atomic Energy Regulatory Board (AERB) of India. Licensing includes compliance regarding pharmaceutical preparations and use of radioactive materials.
A radiopharmaceutical preparation is a medicinal product in a ready-to-use format containing a radionuclide suitable for human use. A kit for radiopharmaceutical preparation encloses a vial containing the non-radionuclide components of a radiopharmaceutical preparation in a sterilized and validated form, to which the appropriate radionuclide is added. Radiopharmaceuticals derived from the kits are intended to be used within 12 hours of preparation.
Click To Continue Reading on Radiopharmaceuticals Market
There are over 40 radioisotopes available for use in different therapies. Technetium-99m is the most widely used radioisotope in the diagnostic procedures. Technetium-99m is used as a radioactive tracer, and can be detected in the body by medical equipment (gamma cameras). It is well suited to the role, as it emits readily detectable gamma rays with photon energy of 140 keV. According to International Atomic Energy Agency, over 80% of the total nuclear diagnostic procedures performed worldwide use Technetium-99 as a radiotracer each year and hence, propels the radiopharmaceuticals market growth.
Radiopharmaceuticals use in Diagnostic and Therapeutic Application fuels growth of the nuclear medicine market
The chemical and physical identity is of vital importance, as during administration it targets certain tissues, binding sites, and biochemical pathways. The market is divided into two major areas, namely, diagnostic and therapeutic. Therapeutic applications of radiopharmaceuticals market include cardiology (myocardial perfusion), oncology (tumor imaging and localization), and neurology (cerebral perfusion). Radiopharmaceuticals such as iodine-131 or iodine-125 labeled m-iodobenzyl guanidine (MIGB) are used to treat pheochromocytoma and neuroblastoma. The therapeutic segment nuclear medicine market can be further sub-segmented on the basis of procedure into beta emitters, alpha emitters, and brachytherapy.
Growth of the radiopharmaceuticals market is projected to witness rapid traction in the near future due to its high potential in diagnosis and detection of diseases related to cardiology, neurology, and oncology. Developed regions are expected to be highly lucrative markets for this technology, due to the high capital investment involved and availability of advanced medical facilities in these regions. Major players in the global Positron Emission Tomography (PET) scanners market are concentrating on enhancing their global and regional presence through strategic operational expansion and collaborations with regional players.
Radiopharmaceuticals are widely used in North America, mainly due to availability of advanced healthcare infrastructure, technological advancements, superior medical facilities, and supportive government support. These are also widely used in Europe due to new procedures for diagnosis of diseases and robust medical infrastructure in the region. Radiopharmaceuticals market is projected to witness rampant growth in Asia due to rapid rise in research work in the sector and rising public awareness.
Request a sample copy of this report:https://www.coherentmarketinsights.com/insight/request-sample/70
Key Venors:
Kimberly-Clark Corporation, Eli Lilly and Company, Lantheus Medical Imaging Inc., Ampio Pharmaceuticals Inc., GE Healthcare, Piramal Imaging, Covidien Plc., Cardinal Health, Iso Tex Diagnostics, Inc., Jubilant DraxImage Inc., Bayer HealthCare Pharmaceuticals Inc., Bracco Diagnostic Inc., Navidea Biopharmaceuticals, Nordion Inc., Spectrum Pharmaceuticals, Inc., Mallinckrodt Plc., Bio-Nucleonics Inc., and IBA-Molecular.

Global Geriatric Care Services Market Trends, and Forecast till 2026
Aging increases the risk of chronic diseases such as diabetes, cardiac disorders, arthritis, and respiratory disorders that require continuous monitoring. Also, aging decreases bone density that makes it a challenge for the geriatric population to continuously manage their medical needs and perform their daily activities. Hence, the need for geriatric care services arises, which to a great extent eases the life of the elderly population. Increasing need for continuous health assistance by the elderly population is driving demand for continuing care.
Elderly population suffering from chronic conditions require frequent physician visits, health monitoring and assistance in performing daily activities. Geriatric care service providers offer end-to-end solutions to the elderly population in need of assistance in order to ease their living. Coordinating with the doctor, assessing any medical or nutritional need, assistance in medical and financial planning, home nursing services, and devising complete medical care plans are some of the services offered by various geriatric care service market players.
Click To Continue Reading on Geriatric Care Services Market
Increasing geriatric population is expected to drive growth of the geriatric care services market
According to the U.S. Census Bureau, 2015, population of age 65 years and above is expected to increase over 60% during 2015 – 2030. It is estimated that the highest growth would be in Asia and Latin America. Africa, on the other hand, is the youngest region as of 2015 and is expected to remain the same in the near future. Therefore, growth of elderly care in respective regions is estimated to follow a similar trend. However, affordability of geriatric care services is expected to hinder growth of the geriatric care services market in emerging economies such as India, Brazil, and China. Therefore, cost-effective products need to be offered by the service providers to attract the relatively cost-sensitive customers in these economies.
Increasing range of services by geriatric care centers to improve the geriatric care services market outlook
Geriatric care centers has evolved from being mere psychological support to nursing care and supervision. Geriatric care centers have well-trained specialists for different geriatric care needs. Services include health planning, problem-solving, health assessment and monitoring, home-care services, and personal care assistance. Furthermore, the providers of geriatric care services help customers to choose the most appropriate insurance policies as per their individual needs. The geriatric care managers also offer counseling and support, crisis intervention, decisions regarding appropriate housing options, money management, and assistance with moving an elderly to or from different types of housing. LivHome Technology+, by LivHome, Inc., addresses the safety issues, enables social engagement, and medication support. The company has LivHome Connect that includes 24 hour Active Remote Care (ARC) from in-house licensed nurses to the patients in need.
Moreover, traditional family support system is weakening, owing to increase number of women in the workforce and increasing migration in search of employment. Therefore, there is a growing need for long-term care facilities for elderly population. Increasing elderly population is also compelling governments to adequately fund healthcare programs and elderly care services. This has further encouraged the establishment of many geriatric care service centers across the globe.
Request a sample copy of this report: https://www.coherentmarketinsights.com/insight/request-sample/88
Growing demand for continuing care in the global geriatric care services market
Geriatric care centers provide services include home healthcare, assisted living facilities, adult day care services, continuing care recruitment, and skilled nursing facilities. Skilled nursing facilities and home healthcare are the major revenue contributors for providers, owing to involvement of more time and workforce in these service types. However, demand for continuing care is increasing rapidly. Elderly population is highly prone to injuries, and therefore require continual care. Also, increasing women labor workforce participation has led to increase in demand for continuing care. The continuing care service type segment in the global geriatric care services market is projected to develop at a CAGR of 4.6% during the forecast period (2016-2024).
Key Vendors:
Brookdale Senior living, Sunrise Senior Living Inc., Senior Care Centers of America, Kindred Healthcare Inc., GGNSC Holdings, LLC, Extendicare, Inc., Home Instead Senior Care Inc., Gentiva Health Services Inc., and Genesis Healthcare Corp

Global Dental Implants Market Trend, Share and Forecast till 2024
With changing market dynamics, companies are emphasizing on product innovation and strengthening of distribution channel to sustain and expand their business reach worldwide. Various macroeconomic factors such as increased geriatric population, growing focus on aesthetics, rise in out-of-pocket spending, and establishment of quality healthcare institutions are expected to bolster sales of dental implant devices worldwide. On an average, over 20% of population in EU is aged over 65 years. Moreover, EU spends nearly 9% of its GDP on healthcare. Increasing consolidation, technological advancement, and company laying emphasis on strengthening distribution channels are some of the key dental implants market trends.
Click To Continue Reading on Global Dental Implants Market
Dental implants are implants in the jaw, placed below the gum line, providing a base for planting artificial teeth or bridge. Dental implants market has witnessed drastic rise in the last few years. A number of socioeconomic and technological factors have contributed to overall growth of the industry. Europe and North America account for 65.9% of global dental implants market revenue in 2016. However, market dynamics are expected to witness a major shift, with Asia Pacific and Latin America emerging as the fastest growing markets for dental implant over the forecast period (2016–2024). This is primarily attributed to aging baby boomers population with high spending power. Lately, the industry is witnessing change in market dynamics post-economic recession of 2008. The industry that was once fragmented is slowly shifting towards consolidation. One such example is the acquisition of Astra by DENTSPLY in 2011.
Cosmetic and dental issues arise as a result of aging. According to National Institute of Dental and Craniofacial Research, geriatric population (aged 65 years and above) have an average of 18.9 remaining teeth, in turn creating a highly lucrative market for dental implants. Technological advancements coupled with high demand for dental implant procedure has resulted in a highly lucrative market characterized by high profit margin for players across the value chain. Advancements in technology allow for implants in people without a proper structured or weak jawbone. In 2015, dental implants accounted for over 15% share of global dental device market revenue.
Among product type, titanium dental implants segment is expected to hold dominant position in the dental implants market over the forecast period. The segment accounted for 44.7% of global market revenue in 2015. However, zirconia dental implants segment is expected to hold dominant position in the market by 2024. Key players are launching mini-titanium dental implants solutions, which are compatible with biologically active drugs that are usually incorporated on the surface of implant to improve osseointegration. The consumers mostly prefer titanium dental implants, as it has longer lifespan. Zirconia, a corrosion free metal, is one of the newly introduced concepts in dental implants market and the zirconia dental implants segment is expected to register highest CAGR over the forecast period.
Europe is expected to dominate the market throughout the forecast period. The region is expected to account for 30.8% of overall market share by 2024. Competition in dental implants market has increased primarily between Straumann and DENTSPLY Implants. According to Europa, over 25% of the population in Europe is expected to fall under geriatric age group by 2020. This coupled with high discretionary income in the region is expected to continue to position the region as a highly lucrative market for dental implants. Germany is one of the key markets for dental implants. The country spends highest per capita income on teeth than any other country in Europe.
Request a sample copy of this report:
https://www.coherentmarketinsights.com/insight/request-sample/89
Asia Pacific, followed by Latin America is expected to register fastest CAGR between 2016 and 2024. Asia Pacific market growth is primarily driven by increasing number of dental implants procedure in China, India, and Japan. India dental implants market is expected to register highest regional CAGR over the forecast period. There are over 5,000 dental laboratories with around 300 dental institutes and 200,000 dentists in India. Increasing growing consumer base along with better economic growth is expected to create lucrative market opportunity for industry players. In 2015, India imported over 77% of dental implant products from Germany, U.S., Japan, and Italy. The ‘Make in India’ campaign initiated by the Indian government is expected to bolster number of manufacturing facilities across various healthcare verticals including dental manufacturing sites in the country.
Key Vendors:
DENTSPLY Implants, Straumann AG, Bicon Dental Implants, Anthogyr, KYOCERA Medical Corporation, Lifecore Dental Implants, Zest Anchors, Implant Innovations Inc, and BioHorizons IPH, Inc., Neobiotech USA. Inc., Sweden & Martina, TBR Implants Group, Global D, and MOZO-GRAU, S.A.

Global Dental Implants Market Trend, Share and Forecast till 2024
With changing market dynamics, companies are emphasizing on product innovation and strengthening of distribution channel to sustain and expand their business reach worldwide. Various macroeconomic factors such as increased geriatric population, growing focus on aesthetics, rise in out-of-pocket spending, and establishment of quality healthcare institutions are expected to bolster sales of dental implant devices worldwide. On an average, over 20% of population in EU is aged over 65 years. Moreover, EU spends nearly 9% of its GDP on healthcare. Increasing consolidation, technological advancement, and company laying emphasis on strengthening distribution channels are some of the key dental implants market trends.
Click To Continue Reading on Global Dental Implants Market
Dental implants are implants in the jaw, placed below the gum line, providing a base for planting artificial teeth or bridge. Dental implants market has witnessed drastic rise in the last few years. A number of socioeconomic and technological factors have contributed to overall growth of the industry. Europe and North America account for 65.9% of global dental implants market revenue in 2016. However, market dynamics are expected to witness a major shift, with Asia Pacific and Latin America emerging as the fastest growing markets for dental implant over the forecast period (2016–2024). This is primarily attributed to aging baby boomers population with high spending power. Lately, the industry is witnessing change in market dynamics post-economic recession of 2008. The industry that was once fragmented is slowly shifting towards consolidation. One such example is the acquisition of Astra by DENTSPLY in 2011.
Cosmetic and dental issues arise as a result of aging. According to National Institute of Dental and Craniofacial Research, geriatric population (aged 65 years and above) have an average of 18.9 remaining teeth, in turn creating a highly lucrative market for dental implants. Technological advancements coupled with high demand for dental implant procedure has resulted in a highly lucrative market characterized by high profit margin for players across the value chain. Advancements in technology allow for implants in people without a proper structured or weak jawbone. In 2015, dental implants accounted for over 15% share of global dental device market revenue.
Among product type, titanium dental implants segment is expected to hold dominant position in the dental implants market over the forecast period. The segment accounted for 44.7% of global market revenue in 2015. However, zirconia dental implants segment is expected to hold dominant position in the market by 2024. Key players are launching mini-titanium dental implants solutions, which are compatible with biologically active drugs that are usually incorporated on the surface of implant to improve osseointegration. The consumers mostly prefer titanium dental implants, as it has longer lifespan. Zirconia, a corrosion free metal, is one of the newly introduced concepts in dental implants market and the zirconia dental implants segment is expected to register highest CAGR over the forecast period.
Europe is expected to dominate the market throughout the forecast period. The region is expected to account for 30.8% of overall market share by 2024. Competition in dental implants market has increased primarily between Straumann and DENTSPLY Implants. According to Europa, over 25% of the population in Europe is expected to fall under geriatric age group by 2020. This coupled with high discretionary income in the region is expected to continue to position the region as a highly lucrative market for dental implants. Germany is one of the key markets for dental implants. The country spends highest per capita income on teeth than any other country in Europe.
Request a sample copy of this report:https://www.coherentmarketinsights.com/insight/request-sample/89
Asia Pacific, followed by Latin America is expected to register fastest CAGR between 2016 and 2024. Asia Pacific market growth is primarily driven by increasing number of dental implants procedure in China, India, and Japan. India dental implants market is expected to register highest regional CAGR over the forecast period. There are over 5,000 dental laboratories with around 300 dental institutes and 200,000 dentists in India. Increasing growing consumer base along with better economic growth is expected to create lucrative market opportunity for industry players. In 2015, India imported over 77% of dental implant products from Germany, U.S., Japan, and Italy. The ‘Make in India’ campaign initiated by the Indian government is expected to bolster number of manufacturing facilities across various healthcare verticals including dental manufacturing sites in the country.
Key Vendors:
DENTSPLY Implants, Straumann AG, Bicon Dental Implants, Anthogyr, KYOCERA Medical Corporation, Lifecore Dental Implants, Zest Anchors, Implant Innovations Inc, and BioHorizons IPH, Inc., Neobiotech USA. Inc., Sweden & Martina, TBR Implants Group, Global D, and MOZO-GRAU, S.A.
Divalproex Sodium Market - Opportunity Analysis 2018 – 2026
Divalproex sodium is a Food and Drug Administrator (FDA) approved anti-epileptic drug used to treat migraine headaches, manic episodes of bipolar disorders, and certain types of seizures. Divalproex sodium is known for its anticonvulsant properties for the treatment of epilepsy. Divalproex sodium comes under three dosage forms: capsule, tablet, and syrup. Divalproex sodium oral tablet is available in brand-names, Depakote ER and Depakote. Divalproex sodium is also used in various combinational therapies.
Click To Continue Reading on Divalproex Sodium Market
The recommended initial dosage of Depakote for the treatment of bipolar disorders is 750 mg daily, whereas for complex partial seizures, initial dosage starts with 10 to 15 mg/kg/day. Also, recommended starting dose for migraine headache is 250 mg twice daily to achieve therapeutic response.
For instance, according to study published in JAMA Psychiatry Journal 2012, in a combined sample of 61,392 adults from 11 countries, the total lifetime prevalence of bipolar disorder-I was 0.6%, and 0.4% for bipolar disorder-II and subthreshold bipolar disorder was 1.4%, yielding a total prevalence estimates bipolar disorder spectrum of 2.4% globally.
Moreover, rising patient pool around the world affected with epilepsy and migraine headaches is also expected to boost demand for divalproex sodium market significantly. For instance, according to the World Health Organization 2018 key facts, around 50 million people were globally affected with epilepsy, making it one of the most common neurological disease, and around 80% of the people with epilepsy live in low- and middle-income countries. According to The Migraine Trust Facts and Figures estimates, the global prevalence of migraine was 14.7% for year 2013, that’s around 1 in 7 people. Furthermore, increasing awareness regarding diseases and various government initiatives to prevent head migraine is expected to propel growth of divalproex sodium market size.
However, black boxed warning given by FDA due to the hepatotoxicity, pancreatitis, and high fetal risk associated with the use of divalproex sodium tablet and other known adverse reactions such as birth defects, neurobehavioral adverse effects, and suicidal behavior are expected to restrain the divalproex sodium market growth. Based on the region, the global divalproex sodium market is segmented into North America, Europe, Latin America, Asia Pacific, Middle East, and Africa. North America, specifically the U.S. divalproex sodium market, holds a major global position among the geographies. Owing to increased government initiatives and rising research and development for the introduction of new product are expected to aid in growth of the divalproex sodium market in the region.
For instance, In 2011 The National Quality Strategy (NQS) was launched as the national strategy for quality improvement in health care, This was led by Agency for Healthcare Research and Quality on behalf of the U.S. Department of Health and Human Services (HHS). However, introduction of generic products by market giants in the region is expected to hamper the market growth in the region. For instance, in 2008, Mylan launched generic of Abbott's Depakote ER in the U.S.
Request a sample copy of this report: https://www.coherentmarketinsights.com/insight/request-sample/1913
Asia Pacific is expected to show significant growth followed by Europe, owing to increased prevalence of epilepsy and head migraine among the population in the region. For instance, according to study published in the Journal of Global Health 2017, showed that lifetime epilepsy prevalence was 1.99% in 1990 and 7.15% in 2015 among the Chinese population.
Key Vednors:
Zydus Pharmaceuticals, Inc., AbbVie Inc., Mylan N.V., Aurobindo Pharma, Dr. Reddy’s Laboratories Ltd., Lupin Limited, Orchid Chemicals & Pharmaceuticals Limited, Sun Pharmaceutical Industries Ltd., Teva Pharmaceutical Industries Ltd., and Abbott Laboratories.
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave, #3200
Seattle, WA 98154
Tel: +1–206–701–6702
Email: sales@coherentmarketinsights.com
Visit our news Website: https://www.coherentwire.com
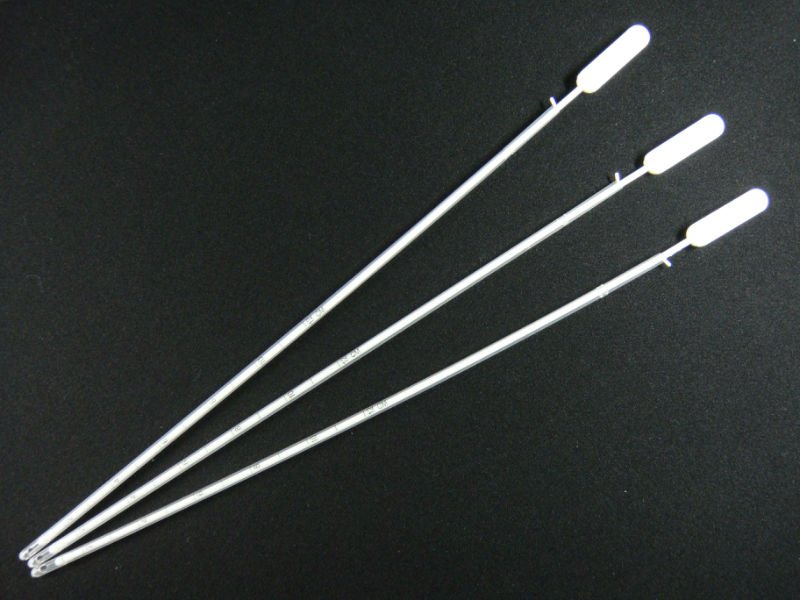
Endometrial Biopsy Cannulae Market Opportunity Analysis, 2018 – 2026
An endometrial biopsy is a procedure used for obtaining small and thin section of endometrial tissue, an inner most lining of uterus, for disease detection and testing. Clinically endometrial biopsy cannulae is used for the evaluation of infertility, amenorrhea, uterine cancer, abnormal uterine bleeding or endometrial infection. Endometrial biopsy cannulae are available in varying size. They are effective and affordable small medical device used for regular diagnostic practice. Disease detection based on biopsy is gaining significant traction, owing to its accuracy, which is expected to drivie the endometrial biopsy cannulae market growth over the forecast period. For instance, according to National Cancer Institute, around 85% of endometrial cancer are diagnosed at earlier stage using endometrial sampling or biopsy. Moreover, increasing incidences of uterine related diseases are driving growth of the endometrial biopsy cannulae market.
Click To Continue Reading on Endometrial Biopsy Cannulae Market
Endometrial Biopsy Cannulae Market Dynamics
Increasing incidences of uterine cancer is the major factor propelling demand for endometrial biopsy cannulae, which in turn is driving growth of the endometrial biopsy cannulae market size. For instance, according to National Cancer Institute’s Surveillance, Epidemiology, and End Results Program, an estimated new cases of cancer to be reported in 2018 are 63,230, in the U.S., which comprises 26.0 cases per 100,000 women.
Furthermore, same source reported an estimated 2.9% of women are expected to have uterine cancer during their lifespan. Therefore, population at high risk of developing cancer frequently undergo cancer diagnosis, which is propelling demand for endometrial biopsy cannulae. According to American Cancer Society’s (ACS) publication, Cancer Facts & Figures 2018, around 92% of uterine cancer are observed in endometrial lining and high prevalence of obesity increases the risk of uterine cancer development proportionally.
Infertility is also diagnosed using biopsy and high prevalence of infertility related issues are also expected to fuel growth of the endometrial biopsy cannulae market over the forecast period. For instance, according to Maternal Health Task Force (MHTF), a project of the Women and Health Initiative at the Harvard T.H. Chan School of Public Health, 2017, nearly 50 million couples experience infertility, worldwide.
Abnormal uterine bleeding is also a significant clinical condition demanding for endometrial biopsy. According to Best Practice & Research. Clinical Obstetrics & Gynaecology, 2016, heavy menstrual bleeding affects 14-25% of women of the reproductive age. Moreover, it states that 800,000 women consult medical professional for abnormal uterine bleeding annually.
Increasing initiatives to create awareness among population for early detection of diseases such as cancer and its importance to reduce the morbidity are expected to boost the endometrial biopsy cannulae market. For instance, governments and non-governmental organizations organize programs to spread awareness about early detection of cancer and its importance and also recommend for the periodic check-ups for cancer. Furthermore, consultation with respect to infertility are increasing at fast pace, owing to increasing awareness, which in turn is propelling the market growth.
However, endometrial biopsy cannulae market growth is expected to be hindered by complications associated with the product use such as uterine or cervical injury, pelvic infection, vagal reaction, incomplete evacuation, or acute hematometra. Moreover, alternative methods for biopsy such as imagining technics or vaginal fine-needle aspiration is also expected to restrain the market growth.
Endometrial Biopsy Cannulae Market - Regional Analysis
On the basis of region, the global endometrial biopsy cannulae market is segmented by Coherent Market Insights into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa regions. North America endometrial biopsy cannulae market holds dominant in the regional segment. The reason attributing to the dominancy of North American market is increasing uterine cancer incidence. For instance, according to National Cancer Institute, the incidence of uterine cancer increased by 1% per year in the time span of 2005 to 2014 in white women. However, Asia Pacific and Africa provides good opportunity for the market players to expand their product range to fulfil the unmet medical needs in low income economies.
Request a sample copy of this report: https://www.coherentmarketinsights.com/insight/request-sample/1937
Key Vendors:
Medline Industries, Inc., CooperSurgical, Inc., MedGyn Products, Inc., Gyneas, Andemed, Nuode, Saipu, Micromed, and Panpac Medical & RI.MOS.
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave, #3200
Seattle, WA 98154
Tel: +1–206–701–6702
Email: sales@coherentmarketinsights.com
Visit our news Website: https://www.coherentwire.com
Triptorelin Market Global Forecast to 2026
Gonadotropin-releasing hormone (GnRH) agonists have greater safety and efficacy profile than estrogens and anti-androgens among various therapies of achieving medical castration. Furthermore, slow-release formulations of GnRH agonists offer patients flexibility, improves quality of life, and eventually reduce cost. Most of the times, prostate cancer progresses with increasing testosterone hormone. Moreover, the production of testosterone can be stopped by surgically removing the testicles or thorough medication therapy. Triptorelin is GnRH agonists which is designed to stop the tentacles from making testosterone. This eventually reduces testosterone levels and minimizes progression of prostate cancer.
Click To Continue Reading on Triptorelin Market
Triptorelin is a decapeptide analogue of Luteinising Hormone-Releasing Hormone (LHRH, also called gonadotropin-releasing hormone [GnRH]). Triptorelin is extensively used in palliative treatment of hormone dependent advanced prostate cancer.
Moreover, according to National Institute for Health and Care Excellence (NICE), in May 2013, the licensed indications for luteinising hormone-releasing hormone (LHRH) agonist: triptorelin were extended to include two new indications for prostate cancer: first in neoadjuvant treatment before radiotherapy in high-risk localized or locally advanced disease and second in adjuvant treatment to radical prostatectomy in locally advanced disease at high risk of progression. Triptorelin is administrated intramuscularly or subcutaneously.
Furthermore, triptorelin is indicated for the treatment of pediatric patients two years of age and older with Central Precocious Puberty (CPP). In July 2017, U.S. Food and Drug Administration (FDA), approved Arbor Pharmaceuticals’: Triptodur, in pediatric patients with central precocious puberty. The approval follows a phase 3 clinical trial (Efficacy, Safety, and Pharmacokinetics [PK] of Triptorelin 6-month Formulation in Patients with Central Precocious Puberty), which examines prepubertal luteinizing hormone levels using triporelin therapy. Study demonstrated a return to pre-pubertal luteinizing hormone (LH) levels in 93% of enrolled patients, with pre-pubertal luteinizing hormone (LH) suppression maintained at 12 months by 98% of patients.
Triptorelin is further being studied to analyze effects of adjuvant hormonal therapy treatments of Tamoxifen, Letrozole and Letrozole + Zoledronate on bone loss in breast cancer patients. According to ClinicalTrials.gov, in July 2017, this study was sponsored by National Cancer Institute (NCI), Naples; and currently present in phase III clinical stage. Moreover, triptorelin market shows lucrative revenue growth in the near future, with the successful completion of this study and potential launch of new products during the forecast period.
Increasing Prevalence of Prostate Cancer among Global Population is expected to Aid in Market Growth
Incensing prevalence of prostate cancer worldwide and wide applications of triptorelin in prostate cancer treatment to limit the cancer progression by reducing the testosterone synthesized by body is expected to drive growth of triptorelin market during the forecast period.
For instance, according to the World Cancer Research Fund (WCRF) International, 2012, over 1.1 million cases of prostate cancer were recorded, accounting for around 8% of all new cancer cases worldwide. Furthermore, around 68% of prostate cancer cases were registered in the developed economies. Highest incidence of prostate cancer was reported in Oceania and North America, while lowest incidence was reported in Asia and Africa.
Moreover, according to the Cancer Research U.K., in 2015, around 47,151 new cases of prostate cancer were registered in the U.K. Furthermore, prostate cancer accounted for 13% of the total cancer cases in the U.K., in 2015. Prostate cancer is the second-most common cause of cancer deaths among males in the U.K. in 2014, accounting for 13% of all cancer deaths among males. In addition, according to the same source, in 2014, around 11,287 prostate cancer deaths were recorded among males in the U.K. The crude mortality rate shows that there were 36 prostate cancer deaths for every 100,000 males in the country.
Request a sample copy of this report: https://www.coherentmarketinsights.com/insight/request-sample/1963
Key Vendors:
Ipsen, Bachem, Ferring Pharmaceuticals Pvt Ltd., Chengdu Tiantaishan pharmaceutical Co., Ltd., Tecnofarma, Dr. Reddy’s Laboratories Ltd., Teva Pharmaceutical Industries Ltd. (Actavis Specialty Pharmaceuticals Co.), Debiopharm Group, and Arbor Pharmaceuticals, LLC.
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave, #3200
Seattle, WA 98154
Tel: +1–206–701–6702
Email: sales@coherentmarketinsights.com
Visit our news Website: https://www.coherentwire.com

Infectious Disease Diagnostics Market to Surpass US$ 25.7 Billion Threshold by 2026 Globally
Infectious diseases are caused by organisms such as bacteria, viruses, fungi, and parasites and are capable of transmission from one species to another species. Diagnosis of these diseases involves identifying the pathogen and its type and the degree up to which the infection has spread. Infectious diseases such as HIV, tuberculosis, malaria, and Influenza are highly prevalent and can be efficiently diagnosed with the help of diagnostic tests available for the respective diseases. Technologies utilized by these diagnostic tests are classified into biochemical techniques, molecular techniques, immunodiagnostics, and conventional culturing, and staining techniques. Biochemical techniques include ELISA, agglutination, and others, while molecular techniques include Polymerase Chain Reaction (PCR) and Next-Generation Sequencing techniques. These tests are capable of diagnosing the infectious diseases accurately with high throughput capacity.
Click To Continue Reading on Infectious Disease Diagnostics Market
Advances in molecular testing techniques have resulted in fast diagnosis of infectious diseases in real-time due to which patients can receive treatment early. Commercially available diagnostic tests include BD Max Smart Cycler, GeneXpert, BD Phoenix CPO detect test, Xpert Xpress Strep A, and BioPlex 2200 Syphilis Total & RPR Assay. Infectious diseases such as HIV, tuberculosis, and malaria are prevalent in emerging economies such as Africa and Asia Pacific. For instance, according to the World Health Organization (WHO), an estimated 36.7 million people were suffering from HIV in 2016.
According to the World Health Organization (WHO), in 2015, an estimated 32,000 deaths from tuberculosis and around 323,000 new cases of TB cases were registered in the Europe. Moreover, around 323,000 incident of TB cases in this region includes 27,000 cases with HIV co-infection.
According to the data published in the Journal of Infection and Public Health, infectious diseases reported the significant causes of deaths in Brazil, in 2016. The high concentration of burden rates observed among men, children under 5 years of age at the coast regions of the state.
According to the Centers for Disease Control and Prevention (CDC), around 5,251 deaths were reported due to influenza infection were registered in 2015. According to the National Ambulatory Medical Care Survey: 2015 data findings, around 16.8 million people visit to physician for treatment of infectious and parasitic diseases in U.S. According to the World Health Organization (WHO), 2016, around 10.4 million people were affected with tuberculosis and an estimated 1 million pediatric population was affected with tuberculosis. Increasing prevalence of diseases would propel the infectious disease diagnostics market.
Individual organizations, manufacturers, and research institutes are forming partnerships, and collaborations to advance research and development of diagnostic tests for infectious diseases. Moreover, funding by individual organizations to advance research is expected to boost the growth of the market over the forecast period. For instance, the University of Virginia’s Global Infectious Diseases Institute funded seven projects designed to tackle challenging problems in infectious diseases worldwide in July 2018. They have formed collaborative teams across diverse academic disciplines to control and treat Zika virus infections and to reduce tuberculosis rates among HIV-infected patients in Africa.
The New York Governor’s Office announced a research collaboration with Regeneron Pharmaceuticals, Inc. to advance the diagnosis and treatment of tick-borne diseases in June 2018. Moreover, Infectious Diseases Society of America (IDSA) and American Society for Microbiology (ASM) released a guide in July 2018, which is expected to help healthcare providers to understand latest technology trends, and knowledge on when to order which test. These factors are expected to affect the growth of infectious disease diagnostic market over the forecast period.
Request a sample copy of this
report: https://www.coherentmarketinsights.com/insight/request-sample/1971
Key Vendors:
Danaher Corporation, Abbott Laboratories, Becton, Dickinson, and Company, F.Hoffmann La-Roche Ltd., Trinity Biotech Plc., Cardinal Health, Inc., BioMérieux, Bio-Rad Laboratories, Inc., AccuBioTech, Co. Ltd., and Acon Laboratories, Inc.
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave, #3200
Seattle, WA 98154
Tel: +1–206–701–6702
Email: sales@coherentmarketinsights.com
Visit our news Website: https://www.coherentwire.com

Hyperphosphatemia Drugs Market Opportunity Analysis, 2018 - 2026
Hyperphosphatemia is a condition caused due to high level of phosphate or phosphorous in the blood. Phosphate is a chemical found in body and contain mineral called phosphorous. Phosphorus mineral is very important for the development of teeth and bones and also convert food into energy for body. Phosphate is mainly found in the teeth, bones, inside the cells, and in very smaller amounts in the blood. Kidney functions for the management of phosphate level in the body. However, various complications/diseases results into impairment of kidney, which leads to chronically high level of phosphate in blood.
Click To Continue Reading on Hyperphosphatemia Drugs Market
Hyperphosphatemia is very common in people with chronic kidney disease more often in people with end-stage kidney disease. Some other possible causes of hyperphosphatemia includes diabetic ketoacidosis, uncontrolled diabetes, high vitamin D levels, and low parathyroid hormone levels.
For instance, in 2016, Astellas Pharma Inc. received marketing approval for its Kiklin Granules 86.2%, indicated for the treatment of hyperphosphatemia in patients with chronic kidney disease in Japan. In 2015, Keryx Biopharmaceuticals, Inc. received approval from European Commission for new drug Fexeric (R) (ferric citrate coordination complex), indicated for the treatment of hyperphosphatemia in adults with chronic kidney disease. In 2014, Keryx Biopharmaceuticals, Inc. received U.S. Food and Drug Administration (FDA) approval for drug Ferric Citrate (formerly known as Zerenex), a new, oral iron-based treatment for dialysis patients with hyperphosphatemia. In 2014, Keryx Biopharmaceuticals, Inc. launched its new drug Auryxia (ferric citrate) tablets. Auryxia is an absorbable iron-based phosphate binder indicated for the treatment of hyperphosphatemia in chronic kidney disease
Strategic merger and acquisition by key players in the market is also expected to create a lucrative environment for the hyperphosphatemia drugs market growth. For instance, in 2013, OPKO Health, Inc. acquired Canada-based Cytochroma Inc., with this strategic acquisition OPKO Health, Inc. has expanded its portfolio with Cytochroma Inc.’s Alpharen, a non-absorbed phosphate binder to treat hyperphosphatemia in dialysis patients.
Furthermore, risk of developing hyperphosphatemia is very high in the people suffering from chronic kidney disease. Hence, increasing prevalence of chronic kidney disease is expected to increase the chances of hyperphosphatemia among population, which in turn is expected to increase demand for hyperphosphatemia drugs and drive hyperphosphatemia drugs market growth. For instance, according to the data published in National Chronic Kidney Disease Fact Sheet 2017, an estimated 30 million people or 15% of U.S. adults are suffering from chronic kidney disease. Chronic kidney diseases were estimated to be more common in Women (16%) as compared to men (13%).
Furthermore, Asia Pacific hyperphosphatemia drugs market is expected to witness significant growth in near future owing to increasing approval of novel hyperphosphatemia treatment drug in key regions such as China, and Japan. For instance, in 2014, Japan Tobacco Inc. and its subsidiary Torii Pharmaceutical Co., Ltd. received manufacturing and marketing approval of Riona Tablets 250 mg from Japanese Ministry of Health, Labor and Welfare. Riona tablet is indicated for treatment of hyperphosphatemia, it is a new phosphate binder containing ferric citrate hydrate as the active pharmaceutical ingredient.
Request a sample copy of this report: https://www.coherentmarketinsights.com/insight/request-sample/1976
Key Vendors:
Keryx Biopharmaceuticals, Inc., Astellas Pharma Inc., Japan Tobacco Inc., Ardelyx, Inc., OPKO Health, Inc., BioLink Life Sciences, Inc., F Hoffmann-La Roche AG, Lupin Limited, Fresenius Medical Care, and Vifor Pharma Group.
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave, #3200
Seattle, WA 98154
Tel: +1–206–701–6702
Email: sales@coherentmarketinsights.com
Visit our news Website: https://www.coherentwire.com

Petri Dish Market Trends, and Forecast to 2026
Petri dish also called as cell culture dishes is common glassware used in research and academic laboratories and hospital in order to hold, transfer, and incubate the sample substance under study. Glass petri dish has lid over it and it majorly used to hold the culture medium of Agar on which bacteria, fungus, and virus are grown, which can be studied for various purpose. Petri dish prevents culturing microorganisms while separating them from airborne contaminates. Petri dish is significant Labware in almost all research efforts, in addition many infectious disease are diagnosed with culture medium method that utilizes petri dish. Majorly glass petri dish are used for various experimental activities. However, polystyrene plastic petri dish are also used according to sample need, as some samples are incompatible or react with glass material.
Click To Continue Reading on Petri Dish Market
Increasing research and development in the field of proteomics and genomics and high prevalence of infectious disease and cancer are the major factors driving growth of the petri dish market. For instance, According to report by the International Federation of Pharmaceutical Manufacturer and Association report published in 2017, pharmaceutical industry invests US$ 149.8 billion on research and development annually. There are more than 7000 compounds in preclinical stage of development as per same source.
High prevalence of infectious disease urinary tract infection, Tuberculosis (TB), Ebola, Sexually Transmitted Disease, and respiratory disease worldwide are expected to drive petri dish market growth. According to World Health Organization (WHO) factsheet in February 2018, in 2016, around 10.4 million people suffered from TB and 1.7 million died from the disease (including 0.4 million among people with HIV). According to same source, Over 95% of TB deaths were reported in low- and middle-income economies. Petri dish based culture medium test are carried out to confirm the presence of disease, which is expected to drive petri dish market growth.
Furthermore, according to 2012, EU Industrial R&D Investment Scoreboard, the pharmaceutical and biotechnology sectors represented 17.7% of business R&D expenditure in the world. Petri dish market is an essential part of any research efforts, which is expected to witness major traction due to increasing research activities by commercial companies and research institutes.
However, Petri dish market is been in mature stage for long with less of innovation and no new introduction from key players. Non culture based diagnostic test are rapidly emerging in the market, which offer fast and reliable results as compared to culture based test that rely on petri dish, which in turn is expected to restrain growth of the market. Furthermore, adoption of the automated liquid handling system and other lab automation tools by large research laboratories may hinder growth of the petri dish market. Various small scale manufacturers in emerging economies create strong competition in petri dish market and also makes petri dish market fragmented.
There is high prevalence of chronic disease such as cancer, cardio vascular disease, and diabetes worldwide, research efforts in developed region has been diverted to personalized medicine to cure this disease. Petri dish market would be supported due to this efforts in finding effective treatment avenues for this disease. Personalized medicine use tools of genomics, proteomics, and cell-based therapies to effectively target disease resulting in greater chance of survival of patient. Petri dish is essential part of most of this process such as cell isolation and cell culture growth. Well established healthcare infrastructure coupled with high adoption rate of new technology for the treatment of cancer in the developed region is expected to boost overall sales of petri dish in regions such as North America and Europe. There are over 200 companies engaged in development of personalized cell therapy products in the U.S. alone
In 2017, the FDA approved 2 CAR-T cell therapy for the treatment of patients up to 25 years of age with B-cell precursor acute lymphoblastic leukemia and adult patients with certain types of large B-cell lymphoma. Such advancement in cell-based therapy needs extensive utilization of petri dish, which is expected to fuel petri dish market growth.
Request a sample copy of this report: https://www.coherentmarketinsights.com/insight/request-sample/1978
Key Vendors:
Aicor Medical, NEST Biotechnology, Thermo Fisher Scientific, Greiner Bio-One, Corning, Phoenix Biomedical, Millipore Sigma, Reinnervate, Narang Medical Limited, Biosigma S.r.l., Pall Corporation
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave, #3200
Seattle, WA 98154
Tel: +1–206–701–6702
Email: sales@coherentmarketinsights.com
Visit our news Website: https://www.coherentwire.com
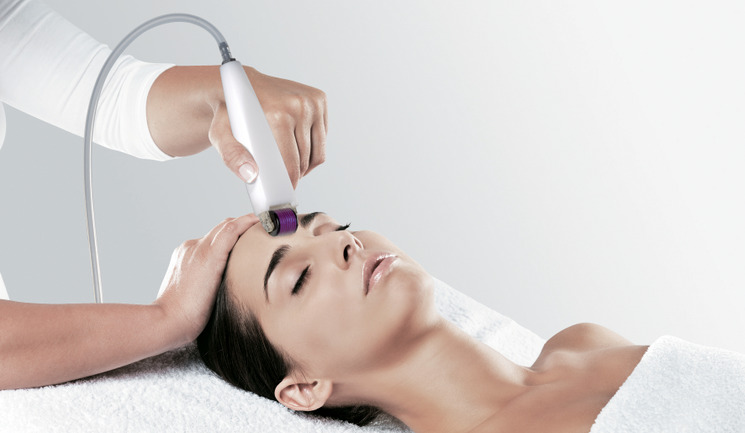
Combination Treatment In Aesthetics Market Forecast to 2026
Combination treatment in aesthetics consists of multiple treatment approaches for improving aesthetic appearance for consumer. It involves both minimally invasive and virtually noninvasive treatment approaches. Combination treatment in aesthetics works on the principle that single modality of treatment cannot offer different advantage each treatment has whereas combination treatment offers best of different treatment. Various treatment approaches under combination aesthetics include, neuromodulators, derma fillers, topical therapies, intense pulsed light photo rejuvenation, laser therapies, radiofrequency based-therapies and ultra-radiofrequency-based therapies.
Click To Continue Reading on Combination Treatment In Aesthetics Market
Combination treatment in aesthetics offers couple of advantages such as reduced number of dermatology clinic visits and improved clinical outcome of treatment, as two different cosmetic procedures contributes its own benefits thereby providing synergic effect. Furthermore, multiple modalities of non-invasive therapies are used in combination treatment in aesthetics.
Combination treatment in aesthetics offers unique approach to treat variety of aesthetic problems such as skin resurfacing, hair removal, hyperpigmentation, scar removal, tattoo removal and other treatments. Increasing awareness about various modalities of aesthetic treatment, preference to improve visual / aesthetic appearance, and decreasing device cost is resulting into wide adoption of the combination therapies from both physician end and consumer end. Increasing number aesthetic procedures for various skin related diseases, to drive growth of the combination treatment in aesthetics market
Increasing incidences of the facial problems (wrinkles, acne, scar, sun damaged skin, stretch marks, and hyperpigmentation) is resulting in to wide utilization of non-invasive as well as minimally invasive cosmetic procedures, which in turn is expected to drive growth of the combination therapies in aesthetics market. Dermatologist are applying the combination of treatment to improve the final outcome with rapid penetration of these procedures. According to the report by International Society of Aesthetic Plastic Surgery titled Global Aesthetic Survey Results published in 2015, around 20 million cosmetic procedures were conducted worldwide, in 2014. This includes 9,645,395 surgical procedures and 10,591,506 non-surgical procedures. According the same source, over 55% of these cosmetic procedures were carried out in 10 countries such as the U.S., Brazil, Japan, Italy, Mexico, Russia, India, Turkey, Germany, and France.
According to Plastic Surgery Statistics Report, 2017, by American Society for Plastic Surgeons, in 2017, 4% increase was observed in patient’s base, which underwent multiple therapies for same cosmetic treatment. Moreover, over 14 million minimally invasive cosmetic procedures were performed that include, botulinum toxin type- A, soft tissue fillers, laser hair removal, laser skin resurfacing, microdermabrasion, and others. Preference for combination treatment is expected to improve with enormous adoption of minimally invasive therapies, which in turn is expected to boost the combination treatment in aesthetics market growth.
However, lack of reimbursement for cosmetic procedures by insurance companies is expected to limit the utilization of the procedure, in turn restraining growth of the market. According to the American Society of Plastic Surgeons (ASPS) in 2017, each session of the non-ablative laser treatments cost around US$ 1,031 per session, while ablative treatments cost around US$ 2,330 per session. Furthermore, no standard guidelines are available for implementing combination treatment in aesthetic, which may lead to slower adoption of this approach.
Need for enhancing facial features would be increasing with increase in aging population, as per analysis by Coherent Market Insights. According to world population prospects report published in 2017, global population is moving toward higher age group. In 2017, an around 962 million people were aged 60 or over, worldwide. This comprised 13% of the global population and is estimated to reach 1.3 billion by 2025 with a growing rate of around 3%, annually. Individual with aging faces skin problems such as decreasing skin fat, wrinkles, hyperpigmentation and other skin related problems. Therefore, combination treatments would be ideal choice in order to enhance facial features as well as to delay facial aging.
Request a sample copy of this report: https://www.coherentmarketinsights.com/insight/request-sample/2066
Key Vendors:
Syneron Medical Ltd., Allegern, Inc., Hologic, Inc., Cynosure, Inc., Merz GmbH and KGaA, Bausch Health Companies, Galderma S.A., Cutera Inc., Alma Lasers, Ltd., Lutronic Corporation, Lumenis Ltd., Cutera, Inc., Strata Skin Sciences, Inc., BISON MEDICAL, Solta Medical Inc., Lynton Lasers Ltd. and Sciton, Inc.
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave, #3200
Seattle, WA 98154
Tel: +1–206–701–6702
Email: sales@coherentmarketinsights.com
Visit our news Website: https://www.coherentwire.com
Peptide Therapeutics in Metabolic Disorders Market Opportunity Analysis, 2017–2025
Peptide therapeutics, due to their various advantages such as high stability, selectivity, safety, efficacy, bioavailability, and tolerability offers wide applications over the other therapeutics classes. Over 7,000 naturally occurring peptides have been identified, which have crucial roles in human physiology, which include hormones, neurotransmitters, growth factors, ion channel, and ligands. The structure of these peptides is usually complex and these conformations depending upon their environment.
Click To Continue Reading on Peptide Therapeutics in Metabolic Disorders Market
Manufacturers are focusing on developments in biologics and biopharmaceutical peptides and proteins for application in diagnostics and therapies. According to the data published in Drug Discovery Today, in January 2015, around 140 peptide therapeutics are currently evaluated in clinical trials. Peptide therapeutics are increasingly been used for therapeutics in metabolic diseases and oncology. Rising incidence of metabolic diseases such as Diabetes mellitus type 2 (T2DM) and Obesity are expected to be factors driving growth of the market. According to World Health Organization (WHO) 2017 data findings, the number people suffering from diabetes have increased from 108 million to 422 million in 2014.
Similarly, the world obesity rate has increased three times since 1975 and in 2016, over 1.9 billion adults (18 year and above) were estimated to be overweight. According to the Centers for Disease Control and Prevention (CDC) October 2017 data findings, in 2011–2012, 78 million U.S. adults had low-density lipoprotein cholesterol levels, which put them at high risk of heart disease and stroke. However, high prices of products is expected to negatively impact the overall market growth.
Among the metabolic disorders, the risk of developing type 1 and type 2 diabetes is related to the combination of variants in multiple genes. In rare cases, patients which are monogenic having single gene mutation which can lead to diabetes. The first peptide to be administrated therapeutically was insulin and has been used in the treatment of diabetes. Development of novel analogues such as lispro insulin that is an injection to be taken prior to meal, has also aided in growth of the market. Another important peptide is glucagon like peptide-1 (GLP-1), which has insulin-releasing properties and also helps to suppress the glucagon level. The glucose dependent action of GLP-1 is more potent to regulate glucose level after a meal. These properties of peptides has aided in development of novel therapeutics and is expected to drive growth of the market in the near future. According to the data published in the January 2015, in Drug Discovery Today Journal, total 60 peptides-based drugs such as viscozyme, pulmozyme, glucagen are available in the market and number of other therapeutic peptides such as cpd86 (preclinical), MOD-6030 (preclinical), MAR709 (II) and semaglutide (III) are currently evaluated in different phase of clinical trial thus, strong pipeline is expected to propel growth of the peptide therapeutics in metabolic disorders market in the near future.
Key players in the market are focusing on collaborations and new product launches, in order to enhance their market share. For instance, Rhythm Pharmaceuticals Inc. is engaged in developing obesity drug called setmelanotide a Melanocortin 4 receptor (MC4R) agonist peptide that is meant to be injected daily to regulate the body energy balance and appetite. Moreover, in June 2017, Rhythm Pharmaceuticals and Camurus AB released the positive initial results from ongoing Phase 1A clinical trial, which evaluated the pharmacokinetics and tolerability of an extended-release formulation of setmelanotide.
The company is also conducting trials for the treatment of extremely rare metabolic disorders such as Pro-opiomelanocortin (POMC) and Leptin receptor (LepR) deficiency obesity. Furthermore, in September, 2017, Zealand Pharma and Torrey Pines Institute for Molecular Studies entered into a research collaboration for multi-target research to identify novel peptide-based products by using Torrey Pines Institute for Molecular Studies (TPIMS’s) peptide libraries. This collaboration will help to develop specialty medicines to treat metabolic and gastrointestinal diseases.
Request a sample copy of this
report: https://www.coherentmarketinsights.com/insight/request-sample/2003
Key Vendors:
AstraZeneca plc, F. Hoffmann-La Roche Ltd, Sanofi S.A., Teva Pharmaceutical Industries Ltd., PolyPeptide Group, Eli Lilly and Company, Bachem Holding AG, Takeda Pharmaceuticals Co. Ltd., Ipsen S.A, Rhythm Pharmaceuticals Inc., and Novo Nordisk A/S.
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave, #3200
Seattle, WA 98154
Tel: +1–206–701–6702
Email: sales@coherentmarketinsights.com
Visit our news Website: https://www.coherentwire.com

Colon and Rectal Cancer Drugs Market Opportunity Analysis, 2018 – 2026
Colon and rectal cancer are often grouped together, as they have many common features. Rate of colorectal cancer is increasing among the millennials in the U.S. The American Cancer Society (ACS) estimates that there will be around 97,220 new cases of colon cancer and around 43,030 new cases of rectal cancer registered in the U.S. in 2018. Moreover, owing to increasing risk of colorectal cancer, in May 2018, ACS updated their guidelines for colorectal cancer screenings and recommended to start colorectal cancer screening at the age of 45 rather than at the age of 50.
Click To Continue Reading on Colon and Rectal Cancer Drugs Market
Increasing incidence and prevalence of colon and rectal cancer, worldwide and increasing research and development studies for colorectal cancer treatment is expected to drive growth of colon and rectal cancer market in the near future. For instance, according to February 2018 data findings of the American Cancer Society (ACS), colorectal cancer is the third most common cancer diagnosed in both men and women in the U.S.
Moreover, according to the Center for Disease Control and Prevention (CDC), in 2014, around 139,992 people in the U.S. were diagnosed with colorectal cancer, including 73,396 men and 66,596 women. Among which, around 51,651 people in the U.S. died due to colorectal cancer, including 27,134 men and 24,517 women. Furthermore, according to 2012 data findings of the World Health Organization (WHO), colorectal cancer is the third most common cancer in men (746,000 cases, 10.0% of the total) and the second most common in women (614,000 cases, 9.2% of the total) worldwide, in 2012. Furthermore, around 55% of these cases occurred in developed regions.
Increasing number of research and development (R&D) studies by government and private healthcare organizations is also fueling growth of colon and rectal cancer drugs market. For instance, the American Cancer Society (ACS) spend US$ 54 million on colon and rectal cancer research involving 92 research grants, till March 2018.
Lack of development in the neoadjuvant/adjuvant pipeline agents for the treatment of high risk colorectal cancer resulted in slower growth of colon and rectal cancer drugs market. For instance, according to the data published in the National Center for Biotechnology Information (NCBI) in April 2016, there is lack of clinical trials of systemic adjuvant, neoadjuvant, and perioperative chemotherapy for the treatment of colorectal liver metastases (CRLM). These offers lucrative opportunity for the key players to develop new treatment therapy using neoadjuvant agents for resected patients.
On the basis of region, the global colon and rectal cancer drugs market is segmented into North America, Latin America, Europe, Middle East, Asia Pacific, and Africa. North America holds dominant position in the colon and rectal cancer drugs market, followed by Europe. This is owing to increasing incidence rate of colorectal cancer in these regions. According to the World Cancer Research Fund International, in 2012, Republic of Korea had the highest rate of colorectal cancer, followed by Slovakia and Hungary. Furthermore, since few years, large number of colorectal cancer incidences were registered in Oceania and Europe region, while it shows lowest incidence rates in Africa and Asia region.
Request a sample copy of this report: https://www.coherentmarketinsights.com/insight/request-sample/2019
Key Vendors:
Amgen Inc., AstraZeneca plc, Bayer AG, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo Company, Merck KGaA, Novartis International AG, Sirtex Medical Limited, Sanofi S.A. Sorrento Therapeutics, and Takeda Pharmaceutical Company Ltd.
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave, #3200
Seattle, WA 98154
Tel: +1–206–701–6702
Email: sales@coherentmarketinsights.com
Visit our news Website: https://www.coherentwire.com

